Crystal Field Theory
Crystal Field Theory: Overview
This topic covers concepts, such as, Crystal Field Splitting Theory, Crystal Field Splitting in Tetrahedral Complexes, Exceptions to CFT Theory & Limitations of CFT Theory etc.
Important Questions on Crystal Field Theory
: [Ti(H2O)6]4+ is coloured while [Sc(H2O)6]3+ is colourless.
: d - d transition is not possible in [Sc(H2O)6]3+
What are the exceptions to Crystal Field Theory (CFT) ?
Crystal Field Theory takes only d-orbitals of a central atom into account. The s and p orbits are not considered for the study.
Crystal Field Theory fails to explain the behaviour of certain metals which cause large splitting while others show small splitting.
Crystal Field Theory does not take into account the covalent character of bonding between the ligand and the central atom.
Crystal Field Theory does not take into account the _____ (ionic/covalent) character of bonding between the ligand and the central atom.
Which of the following is strong field ligand?
Which of the following is weak field ligand?
Which of the following is incorrect?
Which of the following complexes is expected to be colored?
A complex of metal has the following electronic distribution in orbitals,
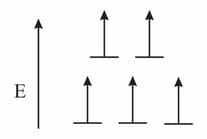
The neutral M has a ground state electronic configuration of Which of the following complexes is consistent with the electronic distribution of (as described above)?
The octahedral complex of a metal ion with four monodentate ligands absorb wavelengths in the region of red, green, yellow and blue, respectively. The increasing order of ligand strength of the four ligands is
In which of the following complexes, magnetic moment will change when all the ligands are replaced by ion to form a cyano complex?
In Wilkinson's catalyst, the hybridization of central metal ion and its shape are respectively:
Which of the following compounds is not coloured?
An octahedral complex with magnetic moment may have:
(a) configuration with weak field ligand.
(b) configuration with strong field ligand.
(c) configuration with strong field ligand.
(d) configuration with weak field ligand.
The complex having the highest spin only magnetic moment is
Crystal field splitting energies for octahedral and tetrahedral geometries caused by the same ligands are related through the expression
The order of octahedral crystal field energy for orbitals are
